Diamonds are one-of-a-kind and there’s no doubt about that. Their formation requires intense pressure and temperature from magma movement beneath the Earth’s surface. But, did you know that there are lab-grown diamonds in existence?
Yes! People have started producing lab-grown diamonds and use them for their industrial characteristics. Some of these characteristics are:
- Hardness
- High thermal conductivity
- High tensile strength
- Wide range of optical transparency
- Resistance to corrosion from chemicals
Because of technological advancements, people manufactured industrial diamonds in the mid-1900s. General Electric (GE) started producing synthetic diamonds in 1954. Optimal temperatures and pressures from deep within the Earth’s crust are duplicated to synthesize lab-grown diamonds.
In this article, we will explain the technology involved in lab-grown diamonds and contrast the real from the synthetic one. Let’s get to it!
Optimal Conditions for Natural Diamonds
Natural diamonds are formed 90 miles (150 km) below the Earth’s surface where temperature and pressure are optimal for the formation of their crystalline structure. The temperature should be at least 2,000 degrees Fahrenheit (1,050 degrees Celsius) to melt carbon to its cubic crystalline structure.
However, if these specific conditions are not met, the carbon atoms will recrystallize into graphite. Graphite and diamond have the same components but they differ in structure, making them exhibit different properties.
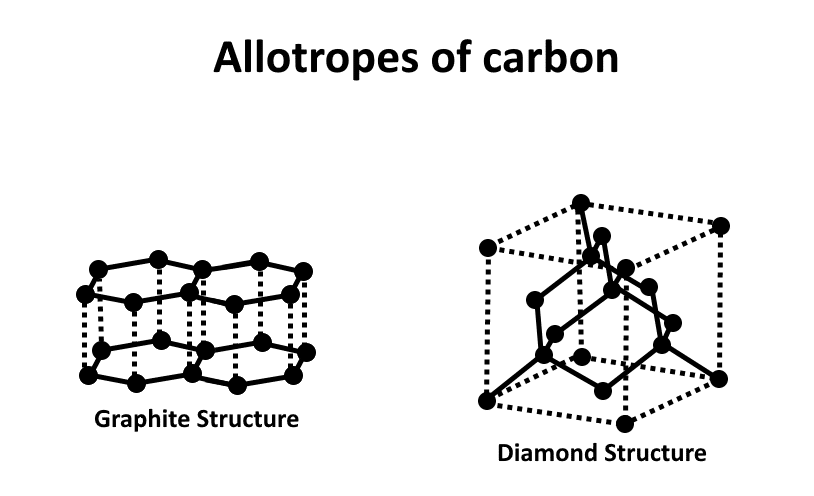
Image source: Wikipedia
Sarp Kaya of the SUNCAT Center for Interface Science and Catalysis experimented with these graphite minerals and transformed them into diamond-like structures. This research proved that we can synthesize diamonds from their allotrope – graphite.
Synthesizing Diamonds: Technologies Involved
First, let me clear this out: lab-grown diamonds are real diamonds. Synthetic diamonds have the same chemical, physical, and visual properties as Earth diamonds. Therefore, the cubic crystalline structure of carbon is called a diamond whether from a controlled or uncontrolled environment.

To synthesize diamonds from a controlled environment, scientists use two methods. These are:
- High Pressure-High Temperature (HPHT) System
- Chemical Vapor Deposition (CVD) Method
Lab-grown diamonds are mainly used for industrial purposes such as diamond drill bits, solar panels, medical equipment, etc. But now, lab-grown diamonds can be polished to achieve a jewelry-grade diamond. However, this process can be expensive and tedious.
If you compare a gem-quality mined diamond to a gem-quality lab-grown diamond, the latter will still be cheaper. It would be a good idea to go for gem-quality lab-grown diamonds for engagement rings. The precise cutting of the diamond determines the beauty and value of the gemstone, whether naturally or synthetically produced.
We’ll discuss how each method can mimic the intense environmental conditions needed to create this precious jewel.
High Pressure-High Temperature (HPHT) System
The HPHT system requires the use of a “diamond seed” to produce industrial diamonds. This “seed” acts as a primer for diamond formation to start. It is placed on a chamber with carbon around the seed and pressurized using different metal presses.
This method of synthesizing diamonds literally mimics the natural way of creating diamonds. The pressure required in the HPHT system is 1.5 million pound-force per square inch (PSI). The temperature should be set to a minimum of 2,500 degrees Fahrenheit or 1,400 degrees Celsius.
The goal is to mimic the growth environment and conditions of the Earth’s crust. This will initiate the formation of the diamond’s cubic crystalline structure from the carbon around the diamond seed.

There are 3 ways to make synthetic diamonds using the HPHT system. These are:
- Belt Press
- Cubic Press
- Split-Sphere Press
The belt press is the one used by General Electric (GE) to produce the first lab-grown diamond. However, due to impurities, these diamonds will never make it to a jewelry store. These are used in the industry for their properties.
The cubic press is mainly used to produce lab-grown diamonds in powdered form. The split-sphere press is the most efficient in producing jewelry-grade diamonds. However, there are still impurities (yellowish color) because the material is exposed to nitrogen during diamond formation. Other impurities include iron, nickel, and cobalt since they are used in the process as well.
Chemical Vapor Deposition (CVD) Method
The Chemical Vapor Deposition (CVD) method mimics the formation of diamonds in interstellar clouds. Instead of subjecting it to extreme pressures and temperatures, the CVD method tones it down a little. Surprisingly, CVD diamonds have fewer impurities than HPHT diamonds.
The CVD diamond growing process includes carbon-rich gasses that are ionized to produce carbon. The vapor deposits form into diamonds when they reach the diamond seed.
First, the “diamond seed” (in this case, a diamond film) is placed in a vacuum chamber. The chamber is filled with hydrocarbon gasses and set to 800 degrees Celsius. Ionizing waves are introduced into the chamber to catalyze the ionization of carbon atoms from the gasses. These free carbons will deposit into the diamond film and will form the diamond layer by layer.
Since this method requires less pressure and temperature, the cost is relatively less. The lab-grown diamonds produced from this method are colorless. Also, there are no metal impurities in this method. Impurities come in the form of graphite and other trace minerals that are trapped in the crystalline structure of the atoms.
How to Tell the Difference?
You can inspect diamonds based on 4 Cs. These are clarity, color, cut, and carat. These properties will determine if the diamond is mined from the Earth or produced from the lab.
Institutes such as the International Gemological Institute (IGI) and Gemological Institute of America (IGA) have grades that determine the quality of the diamonds.
Final Word
Lab-grown diamonds are real diamonds. Even if they are not mined from the Earth, these diamonds have the same visual, chemical, and physical properties.
Natural diamonds are more expensive because they undergo different procedures to attain gem-quality. Lab-grown diamonds, on the other hand, are relatively cheaper because you won’t need to wait for a volcanic eruption to get the gemstone.
If you’re looking for industrial diamonds, then lab-grown diamonds should be your choice. But if you want luxurious jewelry with diamonds, you can go for either mined or lab-grown diamonds. Make sure to remember the 4 Cs when inspecting diamonds.














Leave a Reply