Atoms and elements are basic concepts of Chemistry. The core of this subject lies in understanding these two concepts. So, in this article, let’s focus on Elements vs Atoms.
1. Atom
An atom is the smallest unit of an element. Atoms are indivisible, and these can neither be created nor be destroyed.
The existence of atoms was discovered by the famous scientist John Dalton. An atom is the smallest unit of an element. Atoms combine to form molecules.
An element is a substance that is made up of only one kind of atom.
1.1. Structure of Atoms
An atom is made up of three basic particles:
- Protons
- Neutrons
- Electrons
Atoms have different properties based on the arrangement of these particles. The outermost region of an atom is made up of electrons. The centre of the atom is the nucleus which contains the protons and neutrons. Each particle is identified based on its charge.

The Structure of an Atom
Charge of Atomic Particles:
- Electron has a negative charge
- Proton has a positive charge
- Neutron has no charge
Most of the atoms contain all three particles except Hydrogen(H). It has one proton and one electron but no neutron.
Mass Number: Mass number of an atom is the sum of protons and neutrons in it.
Atomic Number: Atomic number is the number of protons in an atom.
Isotope: atoms with an equal number of protons but different numbers of neutrons are called isotopes.
Atoms lack well defined outer boundaries. It is always described as an atomic radius. Atomic radius is the measure of the distance from the nucleus to the electron in the outer orbit of an atom. On a periodic table of elements, atom size increases when moving down the columns and decreases across rows. The smallest atom is Helium, and the largest atom is caesium.
When an atom or atom has a balanced number of electrons and protons, they are said to be neutral in charge. If they are not balanced, they become charged, and these charged particles are called ions.
Cations
A cation has more protons than electrons. Thus, it has a net positive charge. When the electrons of an atom are pulled away by neighbouring atoms, the negative charge comes down and therefore, the atom gains a positive charge to become a cation.
Anion
An anion has more electrons than protons; when an atom attracts electrons from neighbouring atoms, the negative charge of the atom increases. Thus, making it an anion.
2. Elements
An element consists of a single type of atom. An element cannot be broken down into any other substance. An atom is the fundamental building block of an element. Common examples are iron, copper, silver, oxygen, nitrogen, carbon, etc.
There are ninety-four natural elements and twenty-four synthetic elements. Thus, a total of 118 elements have been identified so far. Elements with atomic numbers below or equal to 94 are seen in nature. Those elements with atomic numbers above 94 are made artificially.
Elements can be classified as metals, non-metals, metalloids and noble gases.
2.1. Metals
Most of the elements known to man are metals. For example, Gold, Silver, Aluminium, Copper, Zinc and Calcium.
- The most commonly seen metals on the earth’s crust are aluminium, iron, calcium, sodium, potassium and magnesium.
- Most of the metals are found in their ores. But a few such as copper, gold, platinum and silver are found in their free state as they do not readily react with other metals.
2.1.1. Properties of Metals
- Metals are solids. But there is an exception of mercury which is a liquid metal.
- Metals can be ductile, which means they can be stretched into thin wires.
- Metals are malleable, which means they can be beaten into sheets, but they won’t break. However, there is an exception of zinc which breaks into pieces.
- Metals have high density.
- Metals are shiny and hard. But there is an exception of sodium which is a soft metal.
- Metals are good conductors of electricity except for tungsten which is a poor conductor.
- Metals are good conductors of heat.
- They have high melting and boiling points.
- Metals are sonorous substances which means when they are stuck; they produce specific sounds.
- Metals have very high tensile strength.
2.1.2. Properties of Non-Metals
- Non-metals are not shiny. They have very dull surfaces.
- When compared to metals, non-metals are very less in number.
- Non-metals exist as soft solids or gases. However, bromine is a liquid non-metal.
- Non-metals cannot be drawn into thin wires, which means they are not ductile.
- Non-metals are not malleable as they cannot be beaten into thin sheets.
- Non-metals are mostly brittle in nature.
- Non-metals are bad conductors of heat and electricity.
- Non-metals have low melting and boiling points except diamond and graphite.
- Non-metals are non-sonorous. They do not produce sound when struck.
2.2. Metalloids
A metalloid is an element that has the properties of both metals and nonmetals. Metalloids can also be called semimetals. They show some properties of metals and non-metals. Metalloids are solids. They are boron, silicon, arsenic, antimony, germanium, tellurium and polonium.
2.3. Noble Gases
They are also called Inert gases because they do not react chemically with other elements. They are found in the air only in traces. Noble gases are a good conductor of electricity. They are odourless and colourless.
There are six noble gases: Helium, Neon, Argon, Krypton, Xenon and Radon.
2.4. Periodic Table
A Periodic Table is a tabular arrangement of chemical elements. Each element is denoted by a symbol which is usually the first letter of its name in English or Latin, and it is always written in capital. However, when the first letter of more than one element is the same, the elements are denoted by two letters.
For example, carbon is denoted by C, calcium by Ca and cobalt by Co.
Horizontal rows of the periodic tables are called periods, and vertical columns are called groups.
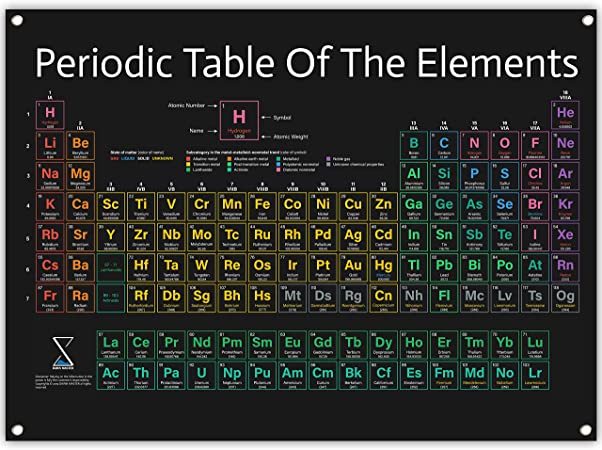
Periodic Table of Elements
Conclusion
Atoms are a part of an element. These are made up of subatomic particles called electrons, protons and neutrons. They are charged particles, and any change in the number of these particles will affect the atom’s charge.
On the other hand, an element is the simplest form of a substance. Elements are made up of only one type of atom. Elements combine to form compounds. Therefore, you can understand further concepts in Chemistry once you know the difference between atoms and elements.












Leave a Reply